.png)

.png)
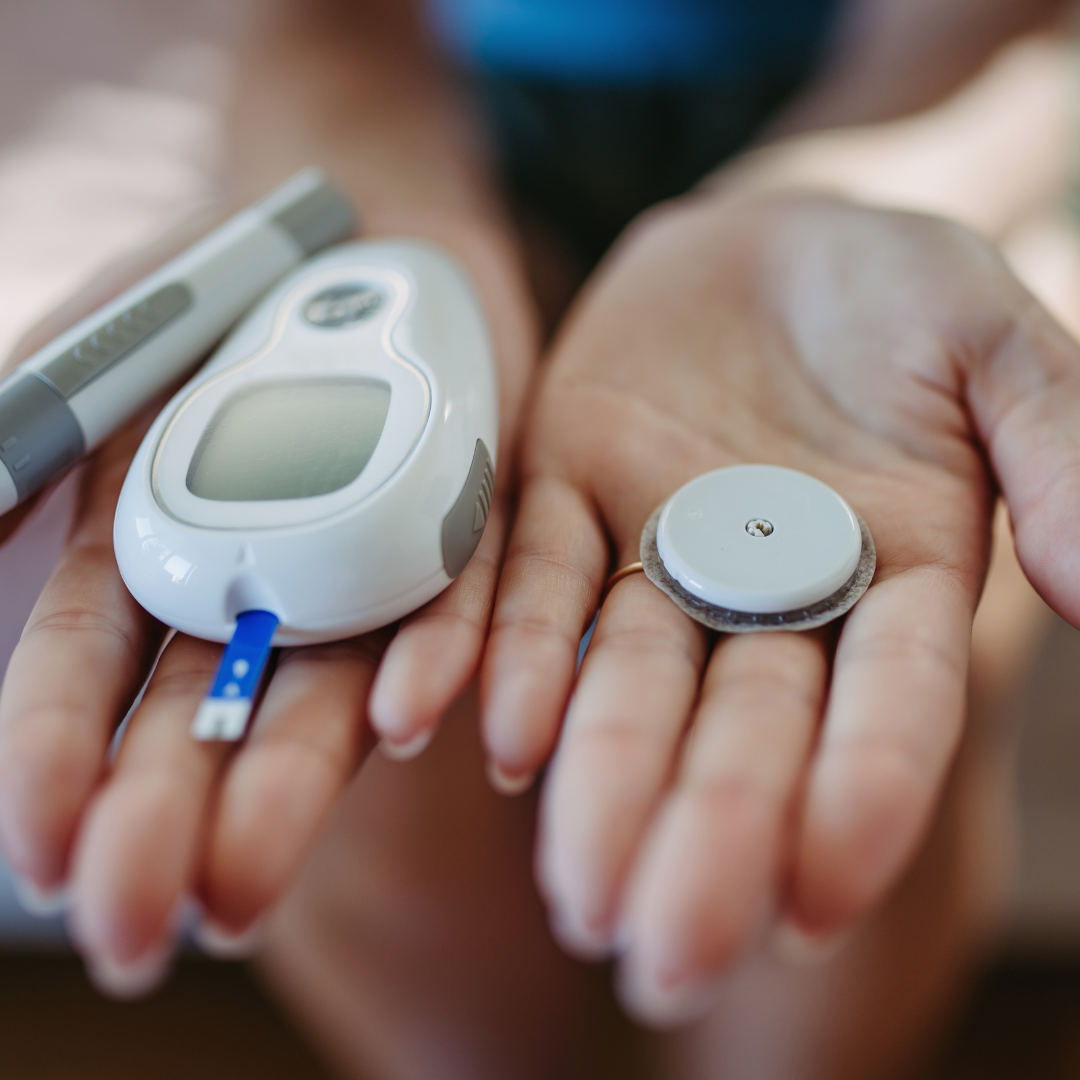
Welcome to Bedard Diabetes Care Department
Bedard's team provides compassionate care and personalized service, including New Patient Setup, Resupply, and Insurance Billing. We process both Pharmacy & Medical benefits, with dedicated insurance verification specialists ensuring a smooth billing process.
Products & Supplies Offered
.png)
CGM Systems & Supplies
Continuous Glucose Monitoring Systems
Sensors & Transmitters
Mobile Integration Tools
.png)
Insulin Pumps & Supplies
Advanced Pump Systems
Infusion Sets
Pump Accessories
.png)
Blood Glucose Meters & Testing Supplies
Blood Glucose Meters
Test Strips & Lancets
Testing Accessories
.png)
Have Questions or Ready to Transfer?
Your Health is Our Priority
Why Choose Bedard
Seamless Transfers
We handle the coordination with your current provider so your care continues without interruption.
Expert Support
Serving New England since 1898, our team brings decades of dependable, compassionate care.
Comprehensive Services
Pharmacy, medical supplies, diabetes care, and more—all from one trusted provider.
Our Process
1. Initial Consultation
We introduce ourselves, confirm details, and explain the process clearly to the patient or caregiver.
2. Insurance & Documentation
We verify coverage, gather needed signatures, and manage paperwork and approvals with your insurer.
3. Approval & Shipment
Once approved, we confirm your order and ship your items directly—quickly and discreetly.
Ongoing Support
Resupply Reminders
Choose how you want to be reminded—email, text, or phone. We make it easy to stay stocked.
Proactive Prescription Management
We monitor expiration dates and automatically request updated prescriptions or authorizations to avoid any gaps.
Comprehensive Diabetes Care Solutions
Insulin Pumps & Supplies
• Tandem t:slim x2
• Beta Bionics iLet
• MiniMed 630G–780G
• Omnipod Systems
Learn MoreBlood Glucose Monitoring
• Blood Glucose Meters
• Test Strips & Lancets
• Testing Accessories
• Ketone Testing
Learn More.png)
Meet Our Specialists
Our specialists are composed of highly skilled and compassionate professionals, each committed to providing exceptional care.

Mary
Mary brings extensive experience in patient advocacy and a deep understanding of the pharmaceutical and medical device industries. This unique expertise positions her as the ideal partner for diabetes healthcare providers, bridging the gap between patient needs and advanced care solutions.
Mary is an active volunteer outside of work, supporting local initiatives that make a difference in the lives of those around her.

Michelle
Michelle has been an integral part of Bedard Medical since 2020, bringing experience in leadership, billing, and healthcare. She currently leads both the Diabetic and Medical Supply Teams, ensuring efficient operations and delivering exceptional service to the community.
Outside of work, Michelle stays busy with her family and finds fulfillment in giving back to local communities. She enjoys cheering at kids' sporting events, supporting local businesses, and exploring Maine’s scenic lakes.
